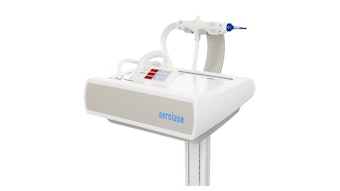
Revelle Aesthetics achieved an extended FDA clearance for its precision cellulite release device, Avéli. The device is now indicated for long-term reduction in the appearance of cellulite in the buttocks and thigh areas of adult females, as supported by clinical data demonstrating treatment benefits through one year of observation.
Related: Revelle Aesthetics Releases New Minimally Invasive Cellulite Reduction Device
"For far too long, millions of women have struggled to feel comfortable in their own skin because of their cellulite and have been settling for less than effective treatment options. Avéli is setting out to change this by finally providing women with a meaningful and lasting solution that targets their cellulite in the areas that have frustrated them the most, their buttocks and thighs," said Caroline Van Hove, president and CEO of Revelle Aesthetics. "Equally as exciting is the role that we believe Avéli will play as a cornerstone in the latest and fastest growing category in medical aesthetics, lower body rejuvenation."
Related: Bimini Health Tech Launches Dermapose Product Line in US & Canada
Avéli is the only minimally invasive procedure that allows a provider to identify which of the fibrous septa bands are causing a cellulite dimple, and then confirm they are releasing those targeted septa to deliver visibly smoother skin.
Avéli is now available nationwide, growing rapidly in plastic surgery and dermatology practices that are focused on the rising needs in body contouring. Revelle's approach to successful integration of Avéli into practices consists of clinical proctoring along with high-touch marketing and educational support.