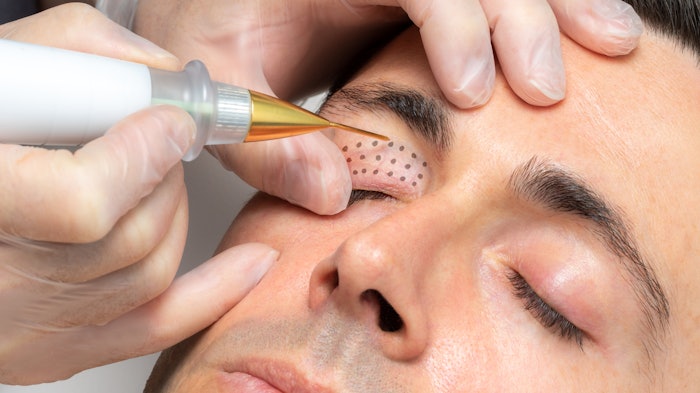
Plasma IQ is an FDA-cleared device indicated for the removal of skin lesions and coagulation of tissue. To test this devices power, pulse duration and needle electrode, researchers John David Holcomb, MD, Rachna Kalhan, PharmaD, and Brian Pilcher, PhD, evaluated skin tissue effects of treatment using Plasma IQ.
Their findings, published in the Journal of Cosmetic Dermatology (March 2022), showed that the Plasma IQ device creates focal microthermal wounds of reproducible depth and width that are similar to other plasma energy skin rejuvenation devices.
Related: High-Energy Helium Plasma Resurfacing Delivers Dramatic Results
Microthermal wounds on pre-auricular and upper eyelid skin from two individual subjects were created using multiple treatment parameters. Researchers assessed the impacts of power, pulse duration and needle electrode type on wound depth, width and thermal spread; they also analyzed the histology to characterize treatment effects.
Related: Cold Plasma Enhances Topical Anesthetic Prior to Laser Treatment
The device's power setting had a statistically significant impact on upper eyelid skin microthermal wound depth and width, but not on thermal spread. There were no meaningful differences in wound width, depth or thermal spread observed based on pulse duration or electrode type.
All microthermal wounds demonstrated dual zones of tissue injury and extended to the superficial reticular dermis. The authors concluded that the microthermal wounds created by the Plasma IQ are of reproducible depth and width and are comprised of dual thermal injury zones similar to other plasma energy skin rejuvenation devices. Device power is the most important factor determining microthermal wound depth and width.