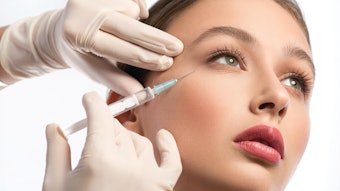
The U.S. Food and Drug Administration (FDA) has approved Restylane Lyft (Galderma) for cheek augmentation and the correction of age-related midface contour deficiencies in patients over the age of 21. The injectable gel (formerly Perlane-L) is now the first and only FDA-approved filler indicated to provide fullness to the midface and to correct and smooth nasolabial folds.
In a clinical trial involving 200 patients, 88.7% of the patients treated with Restylane Lyft showed an improvement in fullness in the right and left midface areas (combined) at two months, and more than half maintained improvement for 12 months. Additionally, 95% of patients reported improvement with the appearance of their midface at two months, and 73% reported improvement at 12 months. The most common adverse events observed in the trial included: tenderness, redness, bruising, swelling and itching, and they decreased in severity over time.
For more information, visit www.restylanelyft.com.
Photo courtesy of Galderma.