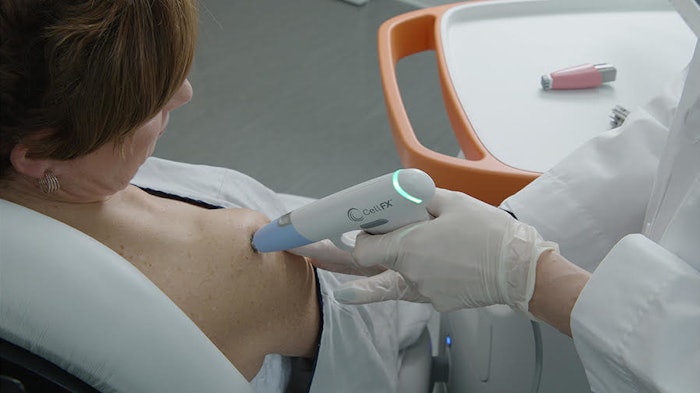
Pulse Biosciences has received the Conformité Européene (CE) mark approval for the CellFX System, a multi-application platform that harnesses the Company’s proprietary Nano-pulse Stimulation (NPS) technology which delivers nano-second pulses of electrical energy to non-thermally clear cells while sparing adjacent noncellular tissue. NPS technology provides the ability to clear unwanted cellular lesions while limiting collateral damage to the surrounding healthy skin, resulting in the potential to clear benign lesions with aesthetically pleasing outcomes.
Related: Triggering Immunity
The CE mark confirms that the CellFX System meets the requirements of the European Medical Devices Directive, which allows Pulse Biosciences to proceed with its planned controlled launch of the CellFX System to medical practices within the European Union (EU) for the treatment of general dermatologic conditions, including:
- Sebaceous hyperplasia (SH)
- Seborrheic keratosis (SK)
- Cutaneous non-genital warts
“We are excited about this major milestone for Pulse Biosciences which keeps us on track to commercialize the CellFX System in the EU this quarter. The CE mark is the first commercial regulatory clearance for the CellFX System and an important validation of the capabilities of NPS technology. Most importantly, it verifies the clinical evidence supporting the beneficial use of the system in these difficult-to-treat lesions. We appreciate all the work and effort from the notified body, scientific investigators and our team that has led to the successful outcome of this process,” said Darrin Uecker, president and CEO of Pulse Biosciences.
In the coming weeks, dermatologist Dr. Afschin Fatemi of Dusseldorf, Germany, will receive training on the first commercial CellFX System.
“I am honored to work with Pulse Biosciences and to be the first dermatologist in the world to offer procedures with the CellFX System using Nano-Pulse Stimulation technology. Across our 13 clinics in Germany, we focus on providing our patients the most innovative and effective aesthetic procedures. I see patients with SH, SK and warts often in my practice and am excited to now have an elegant solution to address these cellular-based lesions,” said Dr. Fatemi, MD, founder of the S-thetic Group.
The prevalence of SH, SK and common, non-genital warts among patients visiting aesthetic dermatologists today is widespread. Based on a 2020 survey among aesthetic physicians from Germany, Spain and France, an average of 200 patients per month who visit aesthetic dermatology practices present with each lesion type (SH, SK, non-genital warts).
“In my extensive investigational work with Nano-Pulse Stimulation technology, I am continually impressed by its cellular-focused ability to fully remove lesions that extend into the dermis without permanently damaging the healthy surrounding dermal foundation,” said Girish (Gilly) Munavalli, MD, Medical Director of Dermatology, Laser & Vein Specialists of the Carolinas and member of the Pulse Biosciences Scientific Advisory Board. “I view this as just the beginning of the clinical potential of NPS technology to clear a broad range of challenging benign lesions with a favorable aesthetic outcome and believe the CellFX System using NPS technology could represent a fundamental change in aesthetic dermatology.”











