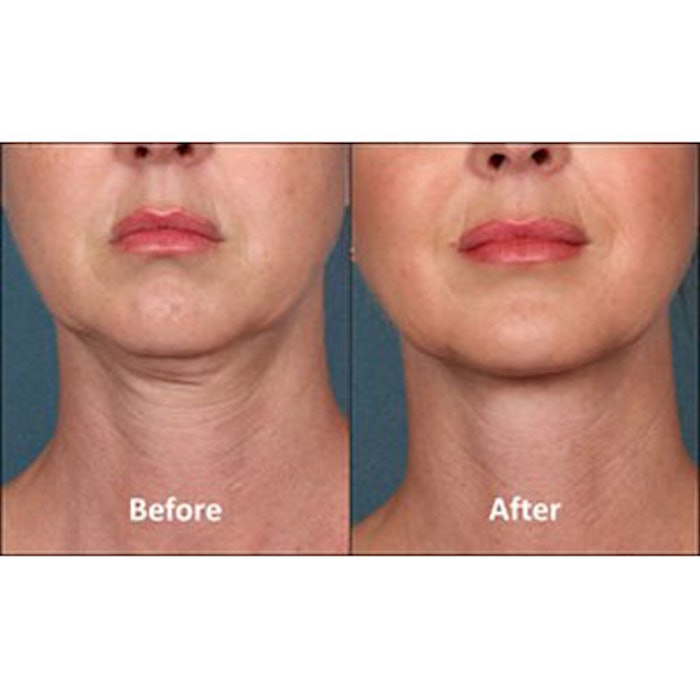
KYBELLA injection (deoxycholic acid, KYTHERA Biopharmaceutcials), also known as ATX-101, has been FDA approved for contouring moderate to severe convexity or fullness associated with submental fat in adults. KYBELLA is a nonhuman and non-animal formulation of deoxycholic acid that aids in the breakdown and absorption of dietary fat. When injected into subcutaneous fat, the drug destroys fat cells. The approval was based on the results of over 20 clinical studies involving more than 2,600 male and female patients.
According to KYTHERA, patients can receive up to 50 injections in a single treatment, with up to six single treatments administered no less than one month apart. Treatment sessions typically take 15 to 20 minutes, and many patients experience visible results after two to four treatments. Once the aesthetic response is achieved, re-treatment is not expected.
The most common adverse reactions in clinical trial subjects were associated with the injection site and included swelling, bruising, pain, numbness, erythema and formation of small areas of firmness around the treatment area. The percentage of adverse reactions reported as mild were 81%, moderate 17.4% and severe 1.6%.
“For the first time, people have access to an FDA-approved, nonsurgical treatment for submental fullness, a condition that can negatively impact the overall appearance of the face and can result in a person feeling older and heavier,” said Keith Leonard, president and CEO of KYTHERA. “With KYBELLA, physicians can offer a clinically proven treatment that is customized to the patient and their treatment goals for an improved chin profile.”
Photo courtesy of KYTHERA.