Gale Force Aesthetics, a national sales and distribution company, has entered into an exclusive sales agreement with Trinnovations, covering the sales of the CuraCator device in the U.S.
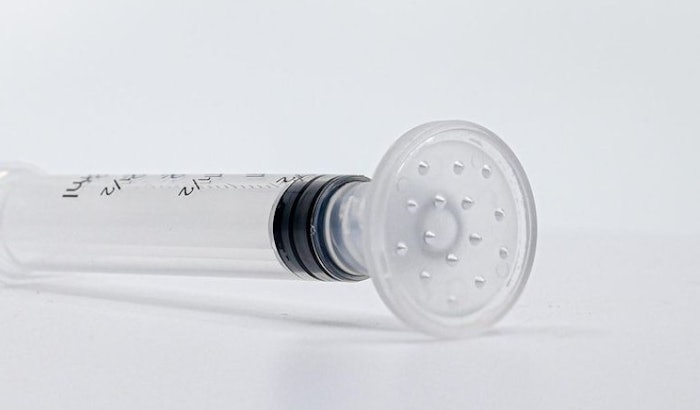
CuraCator was designed and developed by Janine Hopkins, MD, FAAD, board-certified dermatologist. It is a hands-free and needle-free device that allows product, whether liquid, cream or ointment, to be safely and accurately applied to intact or wounded skin.
"CuraCator was developed to improve the safety of patients and healthcare providers by reducing the risks of needle-stick, splatter, waste and contamination of products," stated Dr. Hopkins. "This new standard of care will allow product, whether autologous PRP, serum, cream or ointment, to be safely and accurately applied to patient skin following procedures such as facials, microneedling, surgery or laser resurfacing. Use of CuraCator will effectively alleviate safety concerns and optimize product application from the medspas to the operating room."











