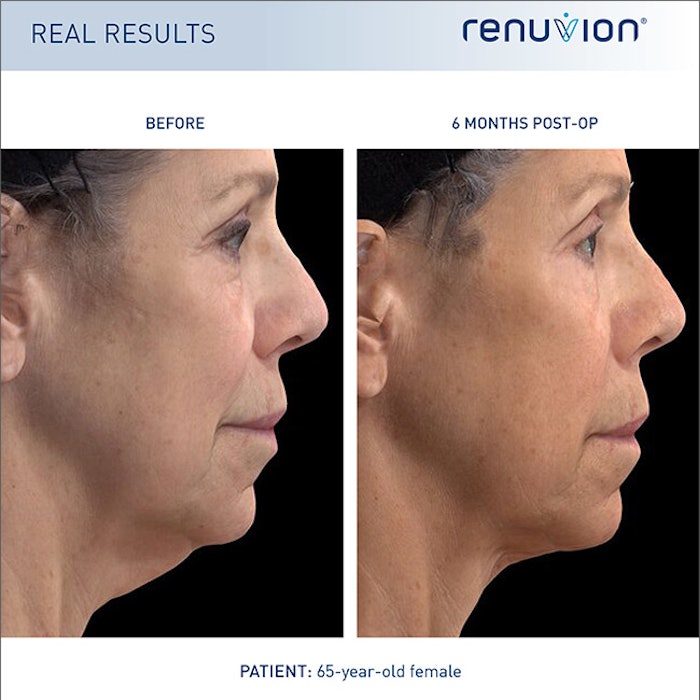
Apyx Medical Corporation announced the FDA approval of the Renuvion APR Handpiece for use in subcutaneous dermatological and aesthetic procedures to contract and improve the appearance of lax (loose) skin in the neck and submental region. The FDA’s approval now mean that Renuvion is the only device on the market that is FDA-approved for contraction procedures to reduce lax or loose skin on the neck and chin.
Related: Apyx To Begin Phase II Trials of Renuvion Plasma Device for Neck and Submental Treatments
“This represents a watershed moment for our Company on our journey to revolutionize the cosmetic surgery market by bringing transformative solutions to our customers. We can now market and sell Renuvion to surgeons and patients for use in the approximately 200,000 neck contouring procedures performed in the U.S. annually,” said Charlie Goodwin, president and chief executive officer of Apyx Medical Corporation. Apyx Medical Corporation is planning on initiating a limited launch around the end of the third quarter of 2022 of the Renuvion APR Handpiece for neck and chin contouring, their goal being to achieve full commercialization by the end of the year.
“We are excited to receive our first regulatory clearance for contouring procedures, and our second regulatory clearance for a specific clinical indication in less than two months, which further demonstrates our Company’s commitment to bringing evidence-based medicine to this industry,” said Mr. Goodwin. He added, “We are excited to make our Renuvion available to surgeons and patients seeking a new, clinically-proven treatment option to improve the appearance of lax, or loose, skin, and look forward to discussing this new clearance in further detail during our second quarter earnings call on August 11th.”











