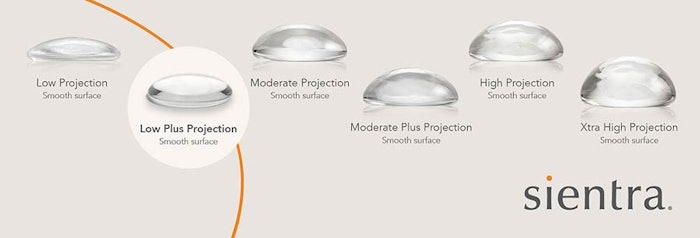
The U.S. Food and Drug Administration (FDA) recently approved the Low Plus Profile Projection Breast Implant made by Sientra, Inc. for use in breast augmentations in women at least 22 years of age or older, in addition to women of any age that are undergoing breast reconstruction. By late July 2022, the product will be available for commercial use in the United States by board-certified and board-eligible plastic surgeons.
Related: FDA Strengthens Safety Requirements for Breast Implants
Sientra’s new Low Plus Profile Projection breast implants will add two new sizing options, 80cc and 110cc gel implants, making them the first implant provider in the country to fulfill and offer this previously unmet need. Sientra’s new product helps bridge the gap “between the low profile and moderate profile implants Sientra already offers,” said Bradley M. Calobrace, M.D., a Kentucky-based board-certified plastic surgeon. Calobrace added, “Now when I utilize Sientra’s new Low Plus Profile Projection Breast Implant, my patients will get the natural look with the upper pole fullness they desire.”
Breast augmentation procedures currently generate the most revenue for plastic surgeons, with over 500,000 women in the U.S. that chose to have a breast augmentation or revision procedure last year alone. According to the American Society for Aesthetic Plastic Surgery, these procedures increased by 41% from 2020 to 2021. As this demand continues to grow, so too does the need for new implant options that allow plastic surgeons better tailor each patient's results to fit their individual preferences.
This newest addition to Sientra’s product portfolio will greatly expand their addressable market and in turn the growth of the company’s market share. Sientra president and CEO Ron Mendez said, “We have long been committed to matching Sientra’s unrivaled safety profile and best-in-industry, Sientra Platinum 20, warranty with a strong R&D pipeline, bringing surgeons and their patients a broader selection for creating their ideal outcomes.”











