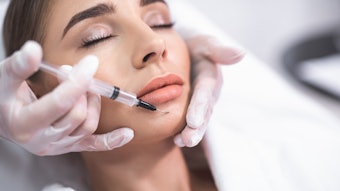
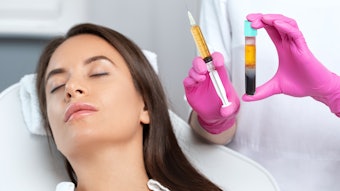
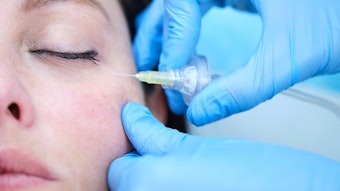
To assess the incidence of delayed-onset nodules from hyaluronic acid (HA) fillers using VYC technology, Jason K. Rivers, MD, FRCPC, FAAD, evaluated patients at a single Canadian Clinic to discover that VYC-associated incidence of delayed-onset nodules was lower than earlier estimates from previous studies.
The study, published in the Journal of Cosmetic Dermatology (May 2022), included 2139 patients with delayed-onset nodules after injections of VYC-20L (57.6%; 2.4 syringes/patient), VYC-17.5L (23.9%; 1.5 syringes/patient) and VYC-15L (18.5%; 1.5 syringes/patient) who were identified by a retrospective chart review.
Related: Reducing Risks of HA Filler-induced Vascular Complications
Of the 2139 patients, seven female patients developed delayed-onset nodules for an overall incidence of 0.33%. Six of those patients reported a potential inflammatory trigger which occured 1-168 days prior to the nodule development. A nodule biopsy in one patient confirmed a foreign-body granuloma.
Prednisone with or without hyaluronidase proved to be the most effective course of treatment, and in two patients, the nodules resloved themselves spontaneously. The incidence of delayed-onset nodules was not associated with injection technique or amount of product used.
Related: Needle vs. Cannula: HA Filler Injections in the Tear Trough
At 0.33%, VYC-associated incidence of delayed-onset nodules was lower previously believed, with the current analysis showing that VYC-15L had a rate of delayed reactions comparable with non-VYC products.