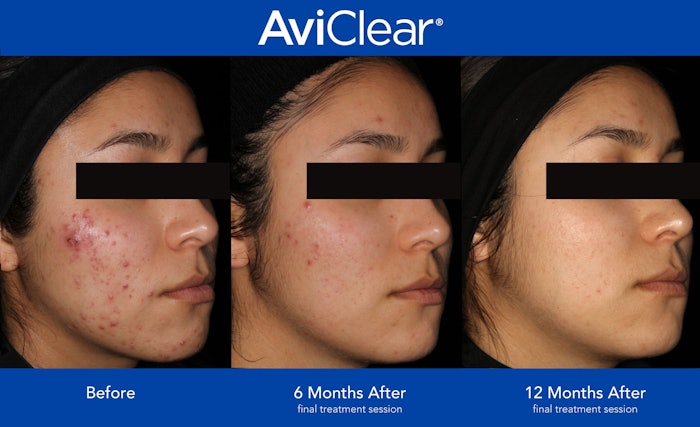
Cutera has announced a new FDA approval of AviClear as a long-term treatment for mild to severe inflammatory acne vulgaris. It is the first acne therapy to claim long-term effectiveness for mild, moderate and severe acne.
Related: Redefine What’s Possible by Treating Acne at the Source
AviClear initially received FDA clearance in March 2022. After months of clinical data evaluation, the FDA has additionally recognized AviClear as a clinically efficacious and proven treatment for the long-term treatment of acne. AviClear selectively targets and suppresses the sebaceous glands, eliminating acne at the source and offering a durable and prescription-free option for patients and providers.
In three, 30-minute treatment sessions cited by Cutera, 90% of patients experienced a visible improvement in their acne six months after their third session of AviClear treatments. Improvement increased to 92% after 12 months of clinical data.
“Those of us who have been using AviClear on our patients since the initial FDA clearance recognized that the results of the treatment get progressively better with time,” said Emmy M. Graber, M.D., MBA, founder of The Dermatology Institute of Boston. “I am thrilled that the FDA has now acknowledged these long-lasting results, giving both patients and dermatology providers greater confidence in the efficacy and durability of AviClear results.”
Sheila A. Hopkins, interim CEO at Cutera, said, “We are proud to receive such a significant and landmark designation. The success of AviClear is a testament to Cutera’s ingenuity and innovation as a pioneering force in results-driven technology. Throughout Cutera’s 25-year history, we have continued to develop devices that offer physicians and their patients breakthrough treatment options, and AviClear is a great example of our game-changing technologies.”











