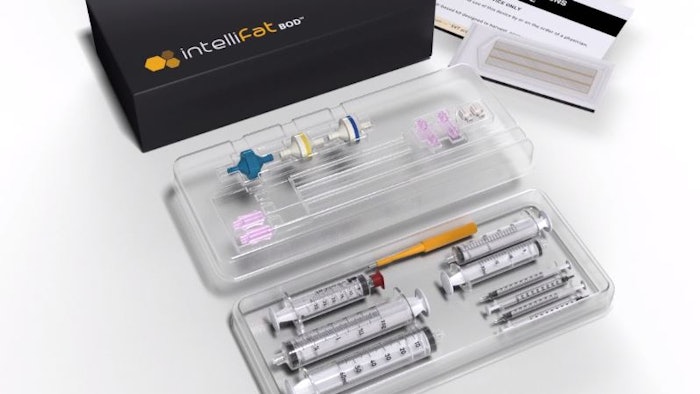
Cellmyx received FDA 510(k) clearance for intelliFat BOD, its single use kit for macro, micro and nano fat tissue harvesting, processing and transfer.
U.S. regulators have approved this medical appliance and technology for use in orthopedic, plastic and cosmetic surgery procedures. intelliFat BOD utilizes a patient's own adipose tissue to aid in patient recovery and healing. In some cases, the appliance is used in conjunction with traditional orthopedic surgery to further advance patient outcomes.
intelliFat BOD Kit
In accordance with the FDA's recent guidelines, the device preserves cellular and tissue micro-architecture of adipose, eliminating residual oil emulsion and blood, thereby providing a tissue byproduct that is minimally manipulated for Human Cell and Tissue Products.
"I have found these systems are cumbersome to use, time-consuming, or produce suboptimal product for injection. But with intelliFat BOD, Cellmyx hit the mark," said Associate Clinical Professor of Medicine of University of Connecticut School of Medicine, Fellow of the American Osteopathic Academy of Sports Medicine, and Board Certified in Sports Medicine & Regenerative Medicine, Dr. Paul D. Tortland, D.O. FAOASM, RSMK. "Their kit is elegantly simple, fast and easy to use, and produces a superlative final product that's easy to inject. Most importantly, I'm seeing outstanding clinical results."
intelliFat BOD kits are sterile, and disposable, and contain components to harvest, process, and transfer autologous adipose tissue for use as an alternative, and/or as an adjunct to surgery for filling soft tissue defects. The technology streamlines procedure times to under 30 minutes, and patients experience no discomfort or downtime during or post-procedure.











