Health Canada has issued a medical device license to Thermi to market its Thermi RF system for the treatment of dermal laxity, making it the first device approved for this indication in the country.
The temperature-controlled radiofrequency (RF) platform technology, which is used for ThermiTight, ThermiSmooth and ThermiRase procedures, was first approved in Canada in 2015 for electrocoagulation of soft tissue and nerve ablation.
“We are excited to announce the first-ever approval of a radiofrequency device for dermal laxity in Canada,” said Vlad Paul-Blanc, president of Thermi. “We are especially pleased to achieve this milestone with impressive clinical data, and we look forward to continuing to expand our offerings in Canada, the U.S. and beyond to ensure physicians and patients always have access to the most cutting-edge treatments.”
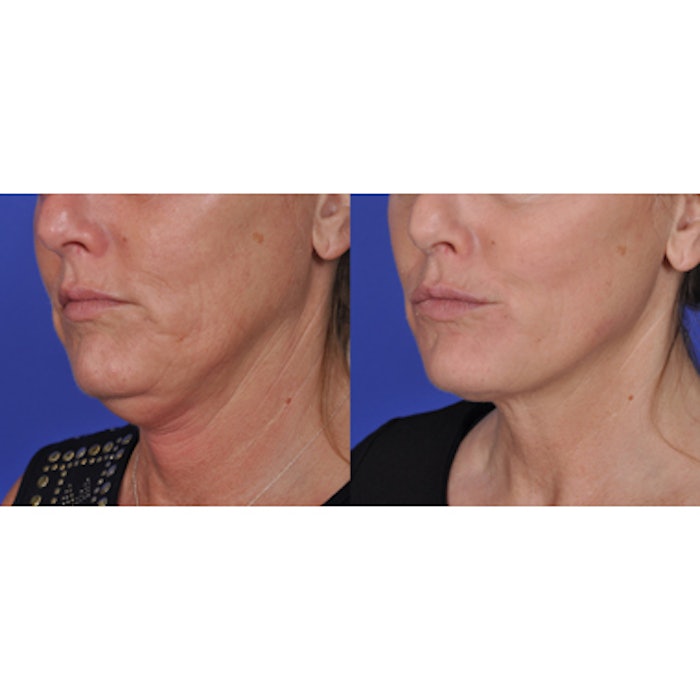
Image: Barry DiBernardo, MD; before and one month after ThermiTight treatment











