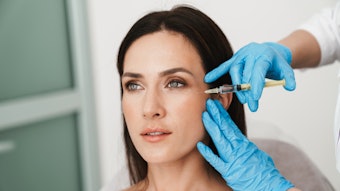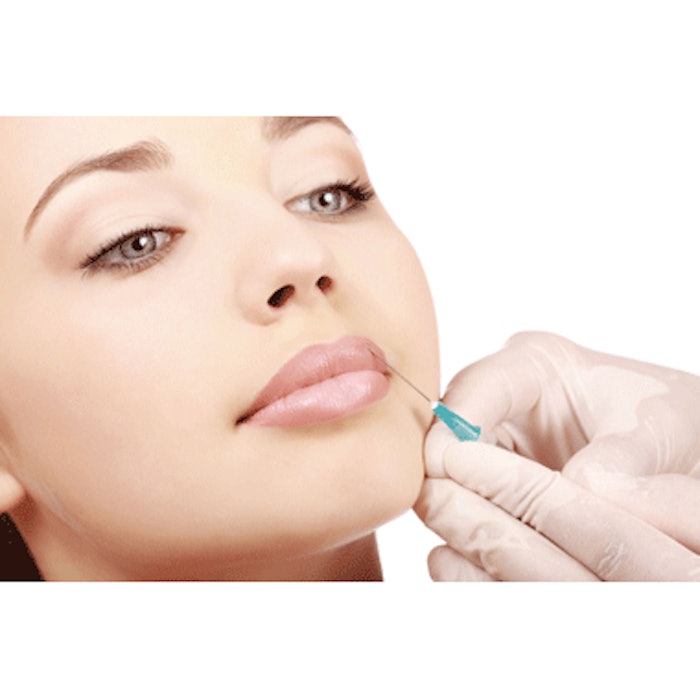
Botox (Allergan, www.botoxcosmetic.com) was first approved in 1989 to treat two medical conditions involving muscle spasm (blepharospasm and strabismus), but since its approval to treat glabellar lines in 2002, it has become much more widely known as a cosmetic drug. That may soon change as more and more research points to the efficacy of botulinum neurotoxin A (BoNT-A) in treating a wide range of skin diseases and conditions.
“Our understanding of these medical applications began with the cosmetic use of botulinum toxin for wrinkle control,” says Erin Gilbert, MD, PhD, a New York dermatologist and assistant professor of dermatology at SUNY Downstate in Brooklyn, New York. “We started noticing changes in surrounding skin, such as smaller pore size and less oil production. We knew from clinical practice that botulinum toxin has an effect on the quality of the skin, but we still wanted to understand how these improvements are occurring.”
Dr. Gilbert, who also has a PhD in neuroscience, is working with Nicole L.Ward, PhD, assistant professor of dermatology, Case Western Reserve University, Cleveland, Ohio, to evaluate botulinum toxin treatments in a variety of skin conditions in a neurology-based laboratory. “Our primary goal is to explore a range of possibilities using a rigorous scientific approach,” she says.
Their most recently published research (“Botulinum Neurotoxin A Decreases Infiltrating Cutaneous Lymphocytes and Improves Acanthosis in the KC-Tie2 Mouse Model,” July 2012 Journal of Investigative Dermatology) showed that a single BoNT-A injection into KC-Tie2 mouse skin significantly improved psoriasiform skin inflammation and epidermal hyperplasia.
Image copyright istockphoto.com
[PAGEBREAK]
Researchers at the University of Minnesota-Twin Cities are currently conducting a related trial on men and women with plaque-type psoriasis. In a preliminary report published online by Dermatologic Surgery (March 2013), they reported improvement in all plaques treated with BoNT-A injections.
Dr. Gilbert is also excited about the application of BoNT-A in diseases characterized by focal itching. “There is a neural component to the mechanism of action for botulinum toxin in these applications,” she says. “Receptors for pain and itch are linked. Preliminary case reports indicate the usefulness of botulinum toxin in stopping itch, but we are now interested in exactly how this works. If we can interrupt the itch-scratch cycle with a single injection session in conditions such as psoriasis, eczema, lichen simplex chronicus or prurigo nodularis than we have found a new treatment option that should last for months.”
In addition, Drs. Gilbert and Ward are beginning to explore the mechanism of action for botulinum toxin in the treatment of herpes simplex, Raynaud’s disease, rosacea and acne.
“From what I’ve seen in my practice and working with other practitioners like Dr. Steven Dayan, one of the best current uses of botulinum toxin for treating skin disease is for patients with rosacea,” says Dr. Gilbert. “We see a rapid rapid reduction in flushing and erythema in patients with erythematotelangiectatic rosacea. For patients with papulopustular rosacea, we also see a significant decrease in the number of active lesions slightly later.”
Yet, botulinum toxin is a deadly poison. Should we be concerned about expanding its uses? “The safety record for BoNT-A is excellent. Problems are generally dose related, occurring most often among patients treated for neuromuscular disorders where as much as 400 units can be injected at one time,” says Dr. Gilbert. “Cosmetic applications usually require less than 80 units, and doses for medical dermatology applications are so small there is no concern at all for safety. For example, to treat rosacea we inject only 10 units. Originally there was some concern about antibody formation, but this has proven extremely rare, occurring in less than 2% of patients across all indications and usually in those that exceed 100-unit doses.”
Linda W. Lewis is a MedEsthetics contributing editor.